Breast cancer deaths disproportionately affect women in low- and middle-income countries. However, healthtech innovations are increasingly making life-saving screening more accessible.
Last year I turned 50, a milestone marked with a fair share of celebrations and a letter from the NHS, the UK’s national health service, welcoming me into its breast screening programme.
At the end of August, only a couple of days after returning from a great summer at home in Spain, I headed to my local London hospital for a routine mammogram. A few days later, I was called back. Before the end of September, following more tests and scans, I was diagnosed with breast cancer.
On 19 October, I unintentionally but fittingly observed International Day Against Breast Cancer by going under the knife. By mid-December, I had both recovered from surgery and completed radiotherapy treatment, all in time for Christmas.
This swift progression of events does not reflect the profound impact of a cancer diagnosis and the physical and emotional challenges of undergoing oncological treatment. Once you know your cells can ‘misbehave’, a certain level of fear remains.
But my doctors and statistics tell me I am now out of the woods, at least for the foreseeable future, and I’m immensely grateful for the system that caught it early, and the science that fixed me.
Global inequalities
Breast cancer is the most common cancer globally and the leading cause of cancer death among women. According to the World Health Organization (WHO), in 2022, 2.3 million women were diagnosed with breast cancer, with 670,000 deaths from the disease registered worldwide.
Deaths from breast cancer disproportionally affect women in low- and middle-income countries (LMICs) – 5-year survival rates in high-income countries exceed 90%, compared to 66% in India, or 40% in South Africa.
In 2021, WHO launched the Global Breast Cancer Initiative (GBCI) with the goal of reducing breast cancer deaths by 2.5% per year, saving 2.5 million lives over a 20-year period.
The target sounds plausible: breast cancer mortality in high-income countries has reduced by 40% over the past 30 years with annual mortality decreases in line with, or exceeding, the GBCI’s 2.5% annual target.
‘Democratising’ access to screening
Late diagnosis of breast cancer can be attributed to various factors, including a lack of awareness, insufficient medical equipment, a shortage of specialised medical staff, and inadequate legislation promoting regular screening.
My tumour was described as a “screen-detected, non-palpable carcinoma”, meaning I couldn’t have felt it by touch or noticed anything unusual by sight. Without that routine mammogram, it could have gone unnoticed for a long time.
Access to breast screening is patchy not just in developing economies but also in high-income countries lacking universal access to healthcare. But international initiatives such as the GBCI, alongside ground-breaking healthtech innovations are resulting in more women having access to live-saving screening, diagnosis and treatment.
Among the companies making strides in breast cancer detection and diagnosis is Mamotest, founded by Guillermo Pepe. The son of a medical doctor running a breast screening clinic in Corrientes, Argentina, Pepe sought to address the frustrations his father often expressed about not being able to reach more women or conduct proper follow-ups.
“Mamotest’s journey started 11 years ago, when healthtech wasn’t even a word,” Pepe explains. With the idea of “democratising” breast screening, he went on to replicate his father’s physical clinic model in other locations, eventually opening 15 centres across Argentina with state-of-the-art mammogram machines which allowed for the digital transmission of images to be interpreted by a radiologist remotely.

The cost of running such an operation, with some machines costing up to $250,000 (€230,000), was substantial, and fundraising proved difficult. “There were no investors focusing on healthcare at the time. It wasn’t appealing to investors as it wasn’t seen as a viable business. They would say, ‘okay, it’s nice what you’re doing, and it has great social impact, but you’re not growing fast enough. It’s not attractive to me’.”
COVID and the healthcare revolution
Pepe describes 2020 as the “year zero for healthtech”, when the world began to realise that much more could be accomplished online beyond banking and e-commerce.
“So, we took that opportunity and started thinking about a solution that was only digital, where we didn’t need to have mammography units installed by us, and the whole operation run by us, which was really challenging and very capital intensive.”
Today, the company offers a fully digital telemedicine solution, connecting clinics and hospitals equipped with mammography units, and providing low-cost, AI-powered diagnostics. It also facilitates communication between patients and healthcare providers, enhancing the overall effectiveness of screening programmes and treatments.
The company secured seed funding of $1.6m from pharma company MSD in 2021. Last year, it raised a further $3.3m from a group of investors including Johnson & Johnson Impact Ventures, to “keep on further developing our platform and connect more centres across Latin America and beyond”, says Pepe.
Portable devices
However, many women living in remote areas still lack access to clinics with the necessary equipment or cannot be reached by mobile units. Thankfully, smaller, more portable screening technologies already exist, and their reliability is constantly improving.
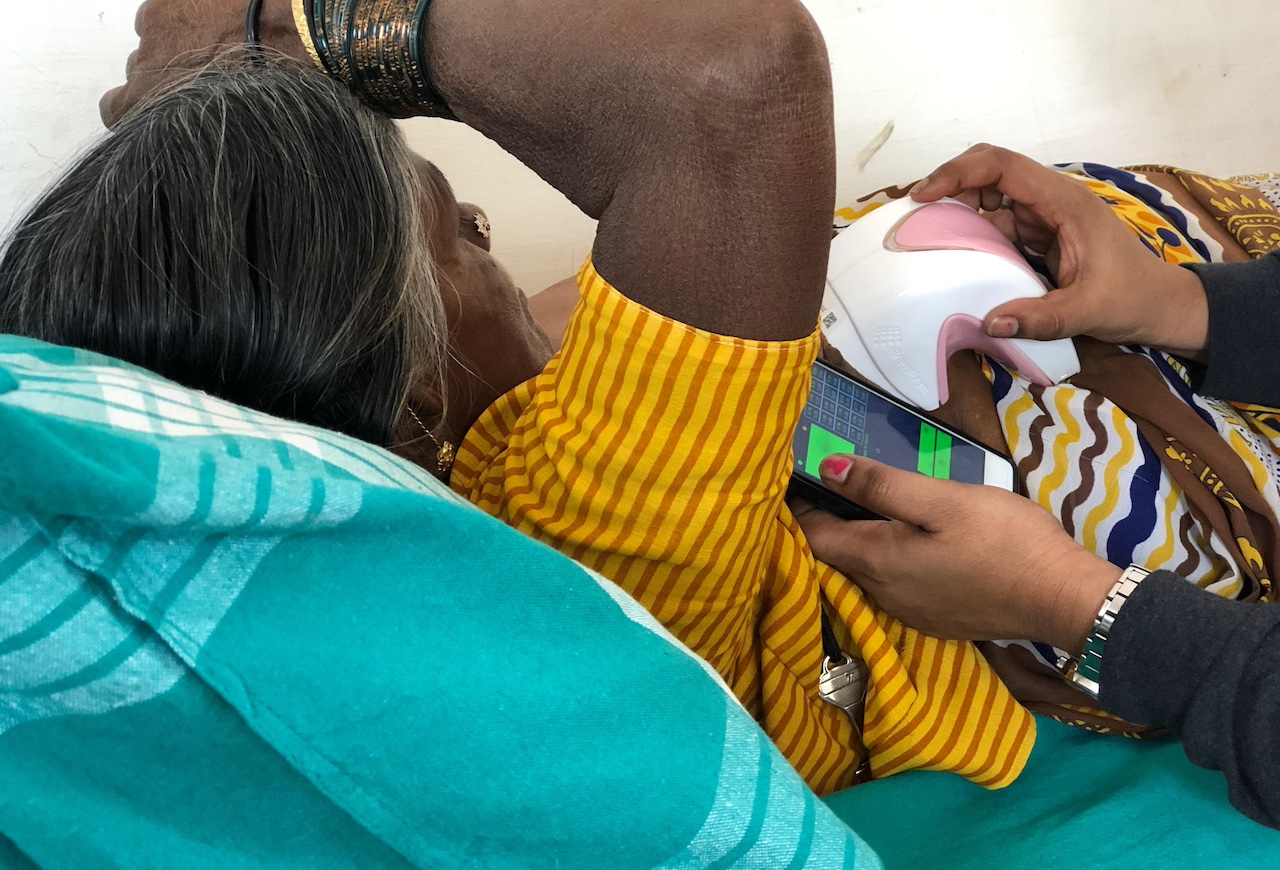
One such technology is iBreastExam, a handheld device that offers quick, painless, and radiation-free breast exams, developed by UE LifeSciences, an American-Indian company which was founded by Mihir Shah, Matthew Campisi and Bhaumik Sanghvi with the aim of providing cost- and clinically-effective solutions for early breast cancer detection in LMICs.
“Mammograms and ultrasounds, including the highly trained staff you need, are very costly and often not available in remote, rural areas where most of the women we need to reach live,” explains Zurich-based Angela Honegger, VP Innovation, UE LifeSciences. “We realised that if we wanted something that’s really scalable in low- and middle-countries, it needed to come at a low cost.”

The device uses “dynamic co-planar capacity sensors” that can detect breast lumps which are stiffer than healthy breast tissue. It can be operated by primary care givers, with the data obtained being shared via a mobile phone app or a cloud-based repository with healthcare providers for effective diagnosis. Mamotest’s network has used the iBreastExam device in remote areas of Latin America.
“The device has a tactile sensor which reacts to the elasticity of the breast tissue to detect areas that are stiffer. It depends on how deep into the tissue the lump is and the breast size, but as a reference, we have detected lumps around 1 centimetre if they’re closer to the surface,” Honegger says.
Although the stage and grade of breast cancer tumours are determined by several factors, a stage 1 tumour is usually less than 2 centimetres across.
Affordability
“We are scanning large populations of asymptomatic women and those who show up with anomalies, around 5%, are sent elsewhere to have further tests. We really can help distribute the resources available in these countries more effectively and make sure women who really need a mammogram are the ones who get it,” she adds.
iBreastExam is used by governments, public and private health providers and NGOs – including Mexico’s state governments, one of India’s leading hospital chains and Egypt’s minister of health, among others – with the cost of each individual examination varying across regions. “We can match whatever affordability means in any given context. So that can go down to $1 or $2 in India, up to $5 to $11 in certain Latin American countries. Basically, we want to match the price of a clinical breast exam provided by a doctor.”
Over 700,000 women have been examined with iBreastExam globally. The company, which received early backing from the Bayer Foundation and has now secured commercial agreements with Siemens and Pfizer, has sold 2 million scans worldwide.
One in eight women will be diagnosed with breast cancer in her lifetime. As daunting as this statistic may seem, early detection makes breast cancer highly treatable. Ongoing innovations and global initiatives should continue to improve breast cancer outcomes worldwide, helping to bridge the gap in survival rates.






